Technological development related to decommissioning
(2019)
QWhat information is needed to safely retrieve fuel debris?
ATo remove debris safely, its mechanical properties should be understood, including the hardness, elastic modulus, and fracture toughness. The research conducted by JAEA focused on evaluating the mechanical properties of fuel debris by atomic numerical simulation. However, more information regarding the properties of fuel debris properties is still required. The developed numerical method can support future experimental analysis of fuel debris properties, this contributing to decommissioning activities at 1F.
Proper removal of fuel debris formed from melting fuel is necessary to the safe decommissioning of the TEPCO’s Fukushima Daiichi NPS (1F). To remove debris safely, its mechanical properties should be understood, including the hardness, elastic modulus, and fracture toughness. As these have not yet been studied in detail, property estimation using simulated debris is essential.
Furthermore, since the detailed components of the debris are not yet completely understood, a vast array of simulated debris must be created and analyzed, thus requiring extensive time and costs. Estimating the mechanical properties of debris with numerical models could therefore greatly reduce costs and help debris removal efforts.
As such, an atomic nuclear model was developed to simulate the mechanical properties of uranium-zirconium oxide (Fig.1), the main component of the fuel debris. A first-principles calculation method was adopted, as this reliable method does not require any empirical parameters.
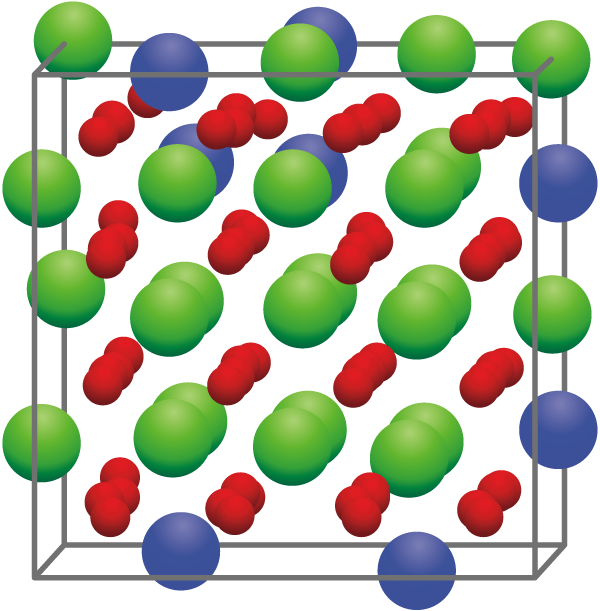
Fig.1 Crystalline structure of uranium-zirconium oxide
This structure, constructed by 24 uranium atoms (●), 8 zirconium atoms (●), and 64 oxygen atoms (●), represents one of the crystalline structures used in the calculations.
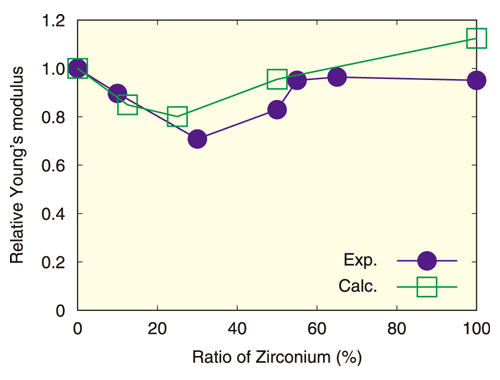
Fig.2 Relative Young’s modulus of uranium-zirconium oxide
Here, the horizontal axis corresponds to the ratio of zirconium atoms to the sum of uranium and zirconium atoms, and the vertical axis shows the relative Young’s modulus of uranium-zirconium dioxide to that of uranium dioxide. The closed circles and open squares represent experimental and calculated results, respectively.
The calculated Young’s modulus, one of elastic moduli, for uranium-zirconium oxides, is shown in Fig.2. Prior experimental work has indicated that Young’s modulus decreases as the zirconium ratio increases until 25 % and increases above 25%. The calculated Young’s modulus showed a similar behavior. Analyzing the simulation results, we found that the distortion of the oxygen atom configuration became the greatest at 25 %, and reduced the Young’s modulus. Thus, this demonstrated another benefit of numerical models: not only can the physical properties be evaluated, but their causes can be elucidated.
Related articles
- Is it possible to visualize radiations? Also, is there a way to understand the situation of contamination inside the Fukushima Daiichi NPS buildings?
- What is a cesium ball?
- How can tritium taken up by animals and plants be measured?
- What happened to the inside of the reactor pressure vessel and storage vessel after the accident?
