Information on treated water and decommissioning
(2024)
QWhat is tritium? What is it like?
ATritium is a family (isotope) of hydrogen. It is also called hydrogen-3. Its half-life is about 12 years, and it emits weak radiation.
Tritium (hydrogen-3) is in the family of hydrogen. It has two more neutrons than hydrogen. It emits weak radiation and changes to helium-3. Helium-3 is stable, and does not emit radiation. The half-life of tritium is about 12 years.
Similar to hydrogen atoms, most tritium atoms bind with oxygen atoms and exist in the form of “water.” A water molecule consists of two hydrogen atoms and one oxygen atom. In water, a tritium replaces a hydrogen atom, and the tritiated water behaves like normal water.
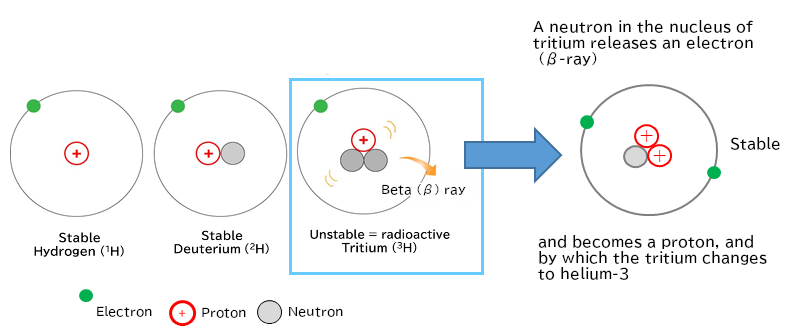
Figure 1 Tritium, an isotope of hydrogen
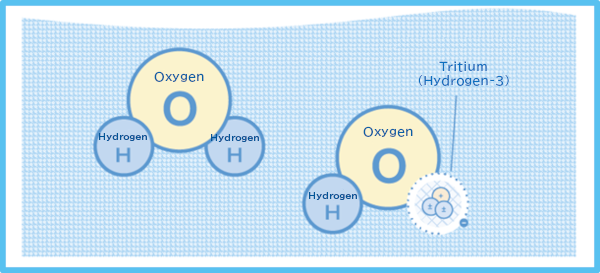
Figure 2 Schematic illustration of water molecules
(Source: PR material issued by the Ministry of Economy, Trade and Industry)
