Radioactivity Dynamics in River System
(2024)
QWouldn't radioactive cesium accumulated at the bottom of lakes, dams, etc. dissolve and affect the ecosystem and farming operations downstream?
AAmmonium ions are generated at the bottom of lakes and dams as a result of the decomposition of organic matter, and it is thought that cesium is dissolved into the lake water through ion exchange between ammonium ions and cesium. The amount of dissolved cesium is so small that the concentration of radioactive cesium in the lake water will increase slightly, but no impact on crops in areas where farming has already resumed has been confirmed.
Reservoir sediments generally act as a sink for radionuclides derived from nuclear accidents, but under anaerobic conditions, several radionuclides remobilize in bioavailable form from sediments to water columns, which may contribute to the long-term contamination of aquatic products.
This study systematically investigated the 137Cs activities of sediment-pore water, providing a direct evidence of the remobilization of bioavailable 137Cs from sediments in a highly contaminated reservoir (Ogaki Dam Reservoir) affected by the TEPCO’s Fukushima Daiichi Nuclear Power Station accident.
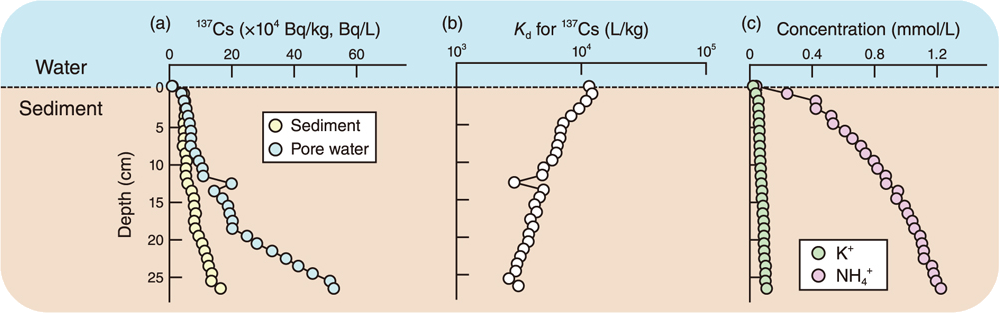
Fig.1 Vertical distribution of 137Cs and water quality in sediment-pore water
Vertical distribution of (a) 137Cs activity concentrations, (b) distribution coefficient (Kd) values for 137Cs in sediment-pore water and (c) water quality in the Ogaki Dam Reservoir in July 2019.
We observed that the dissolved 137Cs activity concentration of pore water (3.0−65.8 Bq/L) was one to two orders of magnitude higher than that of reservoir water (Fig.1(a)). The distribution coefficient (Kd) values for the 137Cs of sediment-pore water ranged from 2.6×103 L/kg to 1.2×104 L/kg and decreased with depth (Fig.1(b)). The major 137Cs competing cation NH4+ (0.24-1.22 mmol/L) was significantly higher than the K+ concentration (0.04-0.11 mmol/L) (Fig.1(c)).
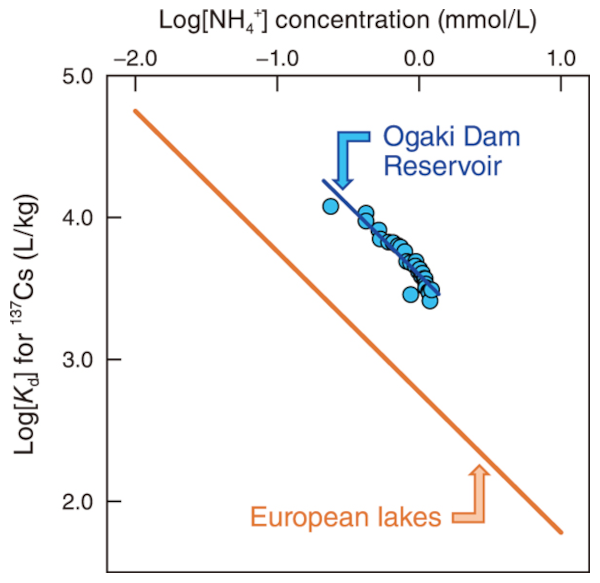
Fig.2 Correlations between the Kd values for the 137Cs and NH4+ concentration in pore water
The blue color line indicates the exponential regression line of the Ogaki Dam Reservoir. The orange color line denote the previously reported exponential regression line in European lakes (Comans, R.N.J. et al.*).
Moreover, the Kd values were significantly and negatively correlated with the NH4+ concentration and the exponential regression lines follow a straight line with an approximate slope close to −1 (Fig.2).
These results strongly indicate that the competitive ion exchange process between 137Cs and NH4+ through a highly selective interaction with the FESs of clay minerals is the major reason for the variability of Kd values between sediments and pore water. However, when compared under similar NH4+ concentrations, the Kd values in Ogaki Dam Reservoir were higher than in some European lakes, suggesting that the sediments of Ogaki adsorb 137Cs strongly and are less likely to remobilize (Fig.2).
Our findings provide important parameter values for mid- and long-term assessments of the radiation impact of radionuclide discharges to freshwater environments.
* Comans, R.N.J. et al., Interpreting and Predicting in Situ Distribution Coefficients of Radiocaesium in Aquatic Systems, Studies in Environmental Science, vol.68, 1997, p.129−140.
Related articles
- Will radioactive contamination continue to accumulate in reservoirs due to the inflow of radioactive cesium from rivers? 【Tendency of the change of the concentration in reservoir beds】
- Will radioactive contamination continue to accumulate in reservoirs due to the inflow of radioactive cesium from rivers? 【Effect of the upstream dam on 137Cs accumulation in the riservoir beds】
- Does radioactive cesium move from the ground surface to underground or into groundwater? 【Movement in the water flow in the litter layer】
- Does radioactive cesium move from the ground surface to underground or into groundwater? 【Movement in underground water】
- How much did the amount or the concentration of Cs released to river water by forest fire? How did them change by time?
