Radioactivity Dynamics in forests
(2021)
QIs the environmental dynamics of radioactive cesium released by the accident at the Fukushima Daiichi NPS any different from that near Chernobyl after the Chernobyl disaster?
AIn terms of accumulation of 137Cs on the forest floor, more than 50% of the initial amount remains within 2 cm of the soil surface in both cedar and deciduous broadleaf forests. The concentration of 137Cs that dissolves in water and runs off these forests is one to two orders of magnitude lower than the concentrations in areas affected by the Chernobyl disaster.
Outside the forests, downward migration and concentration reduction of radioactive cesium progressed faster than in Chernobyl, resulted in a reduction in air dose rates and radioactivity concentration in river water.
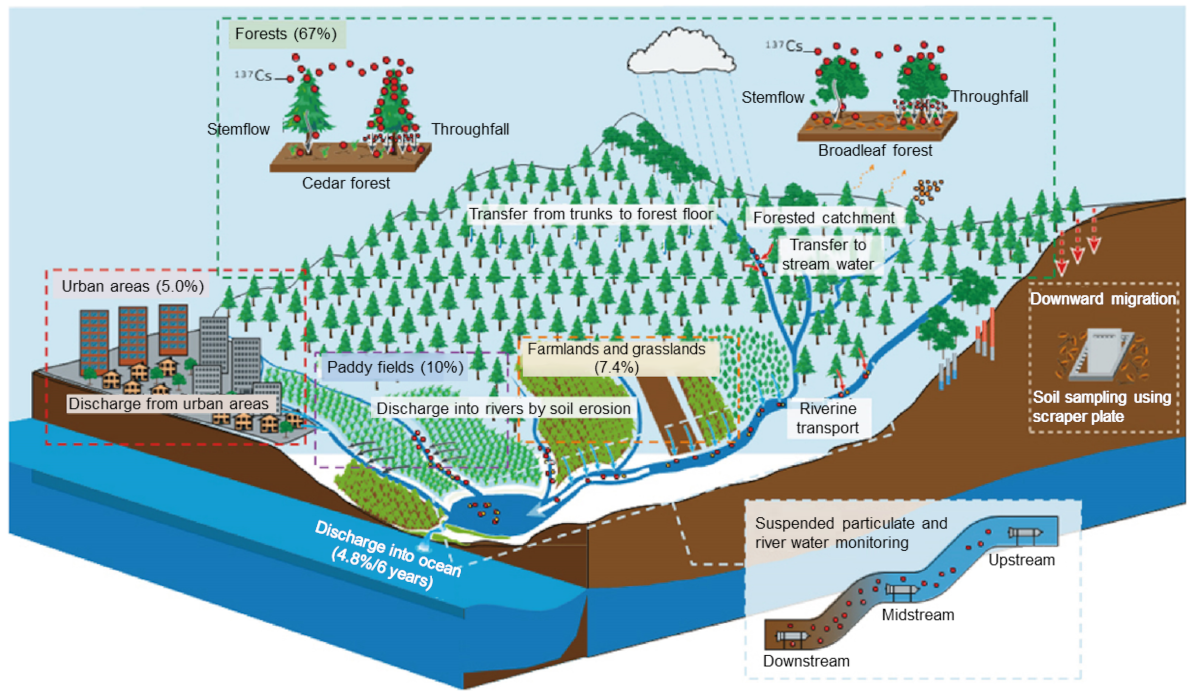
Fig. 1 Schematic illustration of 137Cs migration and monitoring on land
Out of 2.7 PBq of 137Cs fell onto the ground, 67% settled in the forests, 10% in paddy fields, 7.4% in farmlands and grasslands, and 5% in urban areas. Of which, the amount of 137Cs discharged from the ground into the ocean via rivers over six years after the accident was calculated to be about 4.8% (from June 2011 to March 2017, Abukuma River). Taking into account physical decay (13% in the same period) of 137Cs, about 82% of 137Cs that fell onto the Abukuma River catchment still remains.
Accumulation and migration in forests
On the land, forests were the most affected by radioactive cesium (137Cs) released by the accident at the Fukushima Daiichi NPS. Forests accounted for 60% of the affected area (Fig. 1). In forests, radioactive cesium caused various levels of contamination due to factors including direct fallout onto the forest floor, temporary capturing by branches and leaves and transfer to the forest floor thereafter, and the difference in the species of habitant trees. We found that the difference in the species (evergreen coniferous trees or deciduous broadleaf trees) and properties of the forest (e.g., density of trunks, branches, and leaves) affect migration and temporal distribution change of 137Cs in forests, dictating the rate of migration to the forest floor through rainfall and falling leaves and branches (Fig. 2).
In evergreen coniferous trees, the amount of 137Cs accumulated in tree bodies (entire tree including bark, trunk, branches, and leaves) dropped by about 60% in one year after the accident. The amount kept falling after that, down to about 2% of the initial deposition eight years after the accident (Fig. 2b). In deciduous broadleaf trees, the amount of 137Cs accumulated in tree bodies decreased to about 60% of the initial levels in one year after the accident, but after that it started to increase especially in the trunks due to absorption from roots. As a result, an amount equivalent to 91% of the initial deposition was still accumulated even eight years after the accident (Fig. 2f). Regarding the amount of 137Cs accumulated on the forest floor eight years after the accident, we found that 54% of the initial deposition remained within 2 cm of the soil surface for cedar forests and 59% for deciduous broadleaf forests.
As a result of continuous monitoring, it was discovered that the amount of 137Cs discharged from the forested catchment via river water or soil in a year was less than 0.3% of the initial deposition, and almost all of the initial deposition still remains in the forest ecosystem.
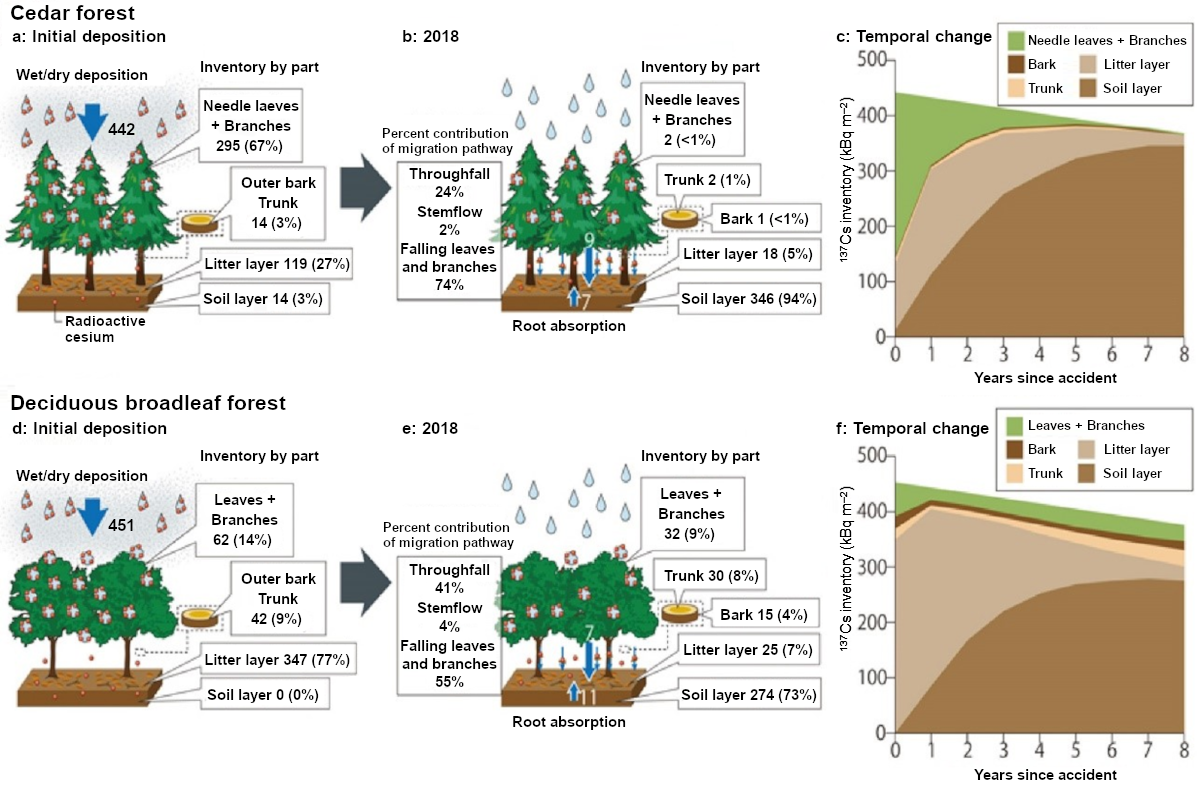
Fig. 2 Migration and temporal distribution change of radioactive cesium in forests
In “Inventory by part” (in kBq m–2), figures in parentheses are percentages to the total inventory. The “Percent contribution of migration pathway” refers to the percent contribution of each pathway to the migration of 137Cs from the tree canopy to the forest floor.
Downward migration in soil
137Cs strongly adsorbs to soil particles. For that reason, immediately after the accident, 99% of 137Cs deposited by the accident existed in the top 10 cm of the soil surface layer. Since soil itself has a radiation shielding effect, air dose rates on the ground are greatly affected by how 137Cs is distributed and migrates in the top 10-cm layer.
The depth distribution of 137Cs in soil can be approximated by an exponential function formula, and the larger coefficient β in the formula means deeper migration. The coefficient β is called relaxation mass depth, and it was found that its value has been gradually increasing, i.e. cesium has been migrating downward, over time since the accident (Fig. 3d). The rate of the downward migration was the highest in abandoned paddy fields with about 1.3 cm per year: after three years after the fallout, the concentration of 137Cs was found to be significantly lower in the surface soil layer and nearly consistent in the range of 10 cm from the soil surface (Fig. 3b). The concentration of 137Cs in cultivated paddy fields was approximately 15% for the depth of 2 cm from the surface as of 2012, exhibiting a significant decrease in the concentration (Fig. 3c).
Meanwhile, the rate of downward migration in undisturbed soil at urban areas was about 0.3 cm per year (about 0.3 g cm–2 by mass depth conversion taking into account the soil density) and affecting a reduction in air dose rates, but the amount of 137Cs that migrated to sections deeper than 10 cm (down to 20 cm) was less than 10% as of 2017. In forests, supply of 137Cs continues due to falling leaves, and 137Cs continued to remain around the soil surface layer (Figs. 3a and 3d).
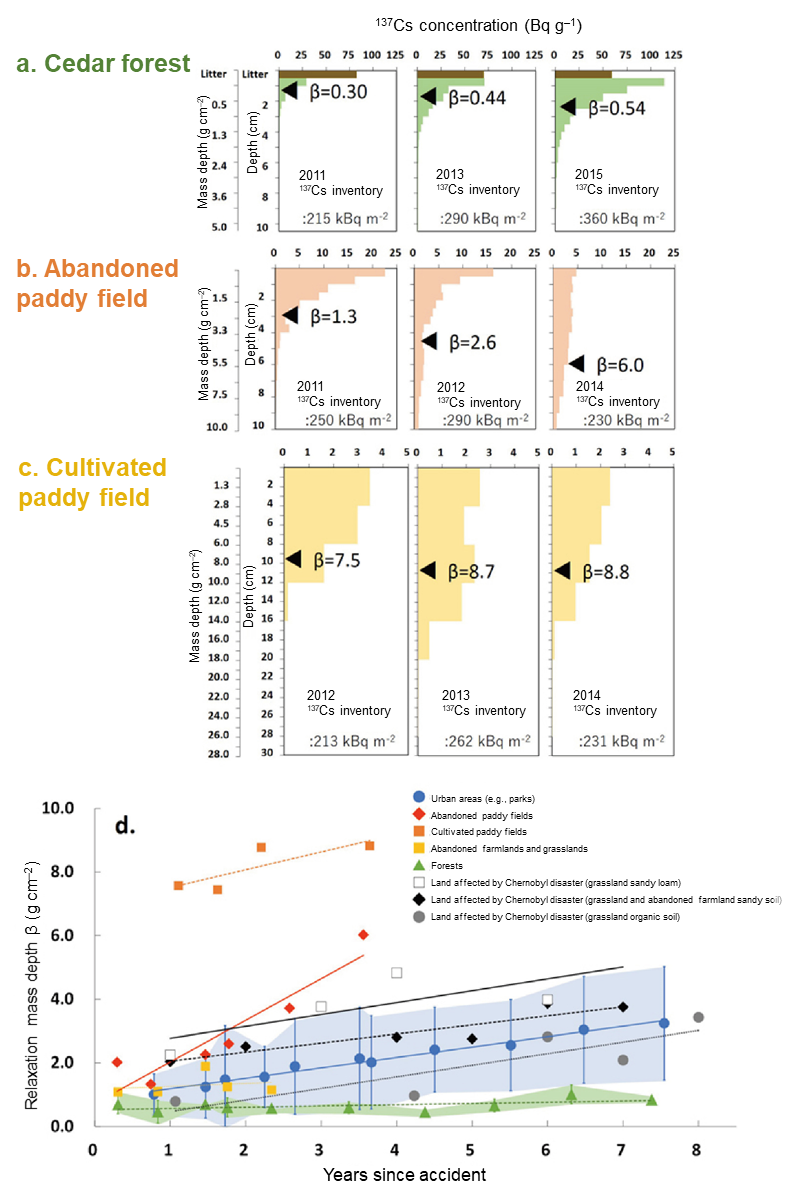
Fig. 3 Temporal change in depth distribution of 137Cs concentration and relaxation mass depth β in various soils
Migration of radioactive materials due to erosion
Measurement of radioactive cesium discharged from paddy fields, farmlands, grasslands, urban areas and forests told us that the concentration of 137Cs that flows into rivers as particulates due to soil erosion varies depending on the type of land use and time elapsed after the accident (Fig. 4).
In paddy fields, while the normalized 137Cs concentration (Sc) was one order of magnitude higher than that in other land use areas for the first six months after the accident, it rapidly dropped after that and became the same level as other land use areas in one year after the accident. For cultivated farmlands, it is likely due to repeated mixing of surface soil (high in 137Cs concentration) with deeper soil (low in 137Cs concentration) by cultivation and washing of 137Cs exposed at the surface. As for discharge from urban areas, the normalized particulate 137Cs concentration was 2.4 times the concentration in the forested catchment as of one year after the accident, but the concentration then rapidly fell and lowered to 1.5 times the concentration in the forested catchment four years after the accident (Fig. 4).
Meanwhile, the normalized concentration of 137Cs discharged from forests did not fall very fast (the rate of reduction was about 60% of that from the urban areas) (Fig. 4).
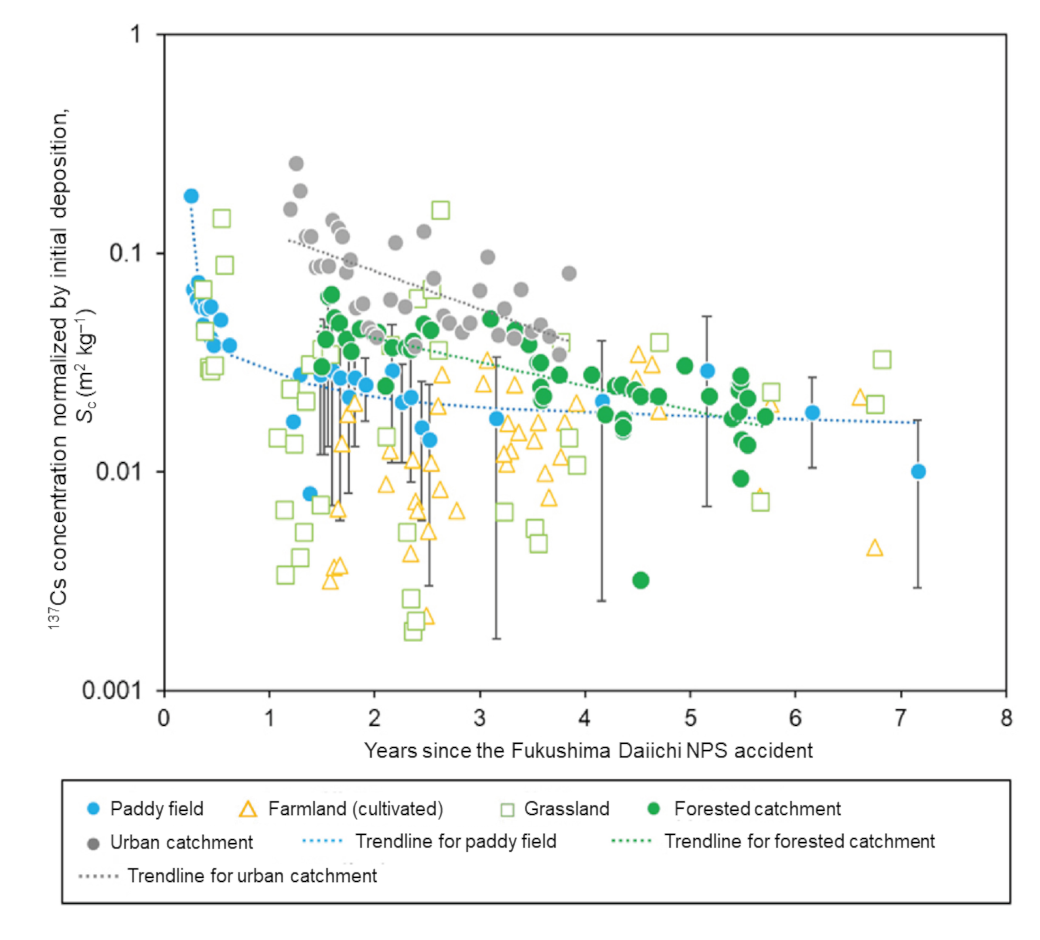
Fig. 4 Temporal change in normalized concentration of 137Cs contained in soil released from various sources after the Fukushima Daiichi NPS accident
Discharge of 137Cs from rivers
Regarding transportation of 137Cs via rivers after the accident at the Fukushima Daiichi NPS, long-term wide-area monitoring has been conducted led by the University of Tsukuba and Fukushima Prefecture. As a result, it was found that the total amount of 137Cs discharged to the ocean via rivers was about 4.8% of the initial land deposition (from June 2011 to March 2017, Abukuma River), of which 96.5% was transported as particulates (Fig. 1).
It was also discovered that land use of the catchment deeply affects the temporal change in the concentration of 137Cs that flows along rivers. Especially, the concentration sharply dropped in rivers with high catchment coverage for the sphere of life (= paddy field, farmland, and urban area (PFU)) in one year after the accident (Fig. 5A). This trend was also observed in the survey for the concentration of 137Cs discharged from paddy fields and urban areas into rivers. After going through a steady gradual reduction trend thereafter, six years after the accident, the particulate 137Cs concentration in the water of Abukuma River became 2% of the figures that were observed immediately after the accident (Fig. 5B).
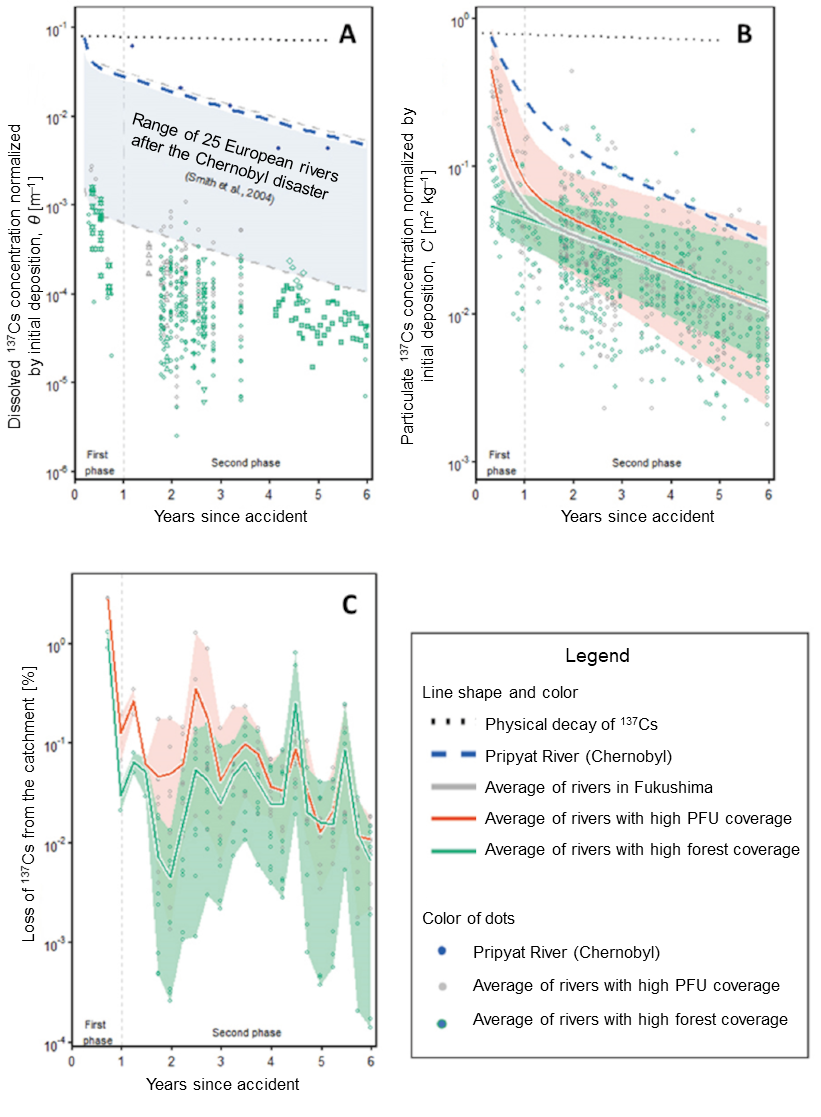
Fig. 5 Temporal change in 137Cs transition in rivers after the accident at the Fukushima Daiichi NPS
- A. Dissolved 137Cs concentration normalized by the initial deposition onto the catchment
- B. Particulate 137Cs concentration normalized by the initial deposition onto the catchment
- C. Loss of 137Cs from the catchment (every four months)
Nuclides other than 137Cs
Nuclides other than radioactive cesium were also released into the environment by the accident at the Fukushima Daiichi NPS. Such nuclides include radioactive strontium (Sr) and plutonium (Pu), which are found in Fukushima, but the quantity is relatively small. For example, the concentration of 90Sr is 1/100 to 1/10,000 of that of 137Cs in soils within the range of 50 km from the Fukushima Daiichi NPS. In addition, it was found that about 7% of Pu found in Fukushima originates from the accident at the Fukushima Daiichi NPS, and the rest comes from atmospheric nuclear tests conducted in the past.
Comparison with the Chernobyl disaster
The natural conditions (e.g., characteristics of forests, land use, topography, precipitation) of Fukushima are different from Chernobyl, and researchers have predicted that the dynamics of 137Cs in the land area would also be different. However, no comparative study has been made to date on the dynamics of 137Cs in these regions based on comprehensive monitoring surveys.
An analysis of the results obtained in this study shows that the rate of 137Cs transition from the tree canopy to the forest floor by rainfall in forests in Fukushima shortly after the accident was higher than that in forests in Chernobyl. This is most likely attributable to higher precipitation in Fukushima (Fukushima: 1200 mm/year, Chernobyl: 650 mm/year). Meanwhile, densely planted artificial cedar forests in Japan have a large amount of biomass in the tree canopy, and the rate of transition would become the same as or slightly lower than Chernobyl in a long-term perspective. 137Cs that transferred to the forest floor by rainfall or falling leaves swiftly migrates downward from the litter layer with time and accumulates in the soil layer. Such cesium does not easily flow out of the system and therefore the dissolved or particulate 137Cs concentration in the river water was one order of magnitude lower than that in forest rivers in European states (Figs. 5A and 5B). In addition, forests in Fukushima are mountainous and have steep slopes, and some of fallen leaves containing 137Cs moved lower along the slopes and Cs was discharged into rivers. Because the majority of discharges into rivers come from mountain streams, as stated above, the annual discharge rate was lower than 0.3% of the initial deposition: only a small amount was being transported out of forests (Fig. 5C).
It was also found, in Fukushima, that the amount of 137Cs exposed on the surface reduced faster than in Chernobyl. This was because Fukushima has steep slopes and high precipitation and saw cultivation at paddy fields and farmlands, human activities in urban areas and decontamination, while the catchment in Chernobyl mostly consists of abandoned farmlands and forests with limited human activities.
For example, the relaxation mass depth β, a parameter that indicates downward penetration of radionuclides, of 137Cs in cultivated paddy fields in Fukushima was two to four times as much as the values in areas affected by the Chernobyl disaster (Fig. 3d). The surface soil is a source of suspended particulates in rivers, and the rate of reduction in the particulate 137Cs concentration in rivers in Fukushima during a period of one year after the accident was about 1.6 times as much as that in the Pripyat River that runs in Chernobyl (Fig. 5B). As for the normalized dissolved 137Cs concentration, rivers in Fukushima continue to show values about two orders of magnitude lower than the values in European rivers after the Chernobyl disaster. This means, the 137Cs concentration in river water in Fukushima has been rapidly dropping (Fig. 5A). Currently, values observed in periodic monitoring are mostly below standard values, but sometimes 137Cs concentrations exceeding the regulatory standard value are observed in freshwater fish, and that is known to be highly correlated to dissolved 137Cs concentration. In this study, similar to the dissolved Cs concentrations, the contamination level of freshwater fish in Fukushima was found to be one or two orders of magnitude lower than that in Europe after the Chernobyl disaster, indicating much faster recovery than as predicted from what we learned from the Chernobyl disaster.
After the Chernobyl disaster, scientists claimed that the reduction in the dissolved 137Cs concentration in European rivers was due to adsorption of 137Cs onto soil particles that gradually becomes stronger. However, this doesn’t appear to be applicable to the case of Fukushima, for the facts that the land use in the catchment directly affects the particulate 137Cs concentration in rivers and the ratio of particulate 137Cs concentration to dissolved 137Cs concentration (Kd) in rivers does not change with time. Summarizing these findings brings us new understanding that, at least in the case of Fukushima, a reduction in radioactive material concentrations in the sources of soil is causing a reduction in the particulate 137Cs concentrations, resulting in a reduction in the dissolved 137Cs concentration.
(The findings were obtained through collaboration with the University of Tsukuba and other institutions.)
Related articles
- Is radioactive cesium in forests dispersed by wind? 【Near forests】
- Is radioactive cesium in forests dispersed by wind? 【Airy places】
- Will radioactive contamination continue to accumulate in reservoirs due to the inflow of radioactive cesium from rivers? 【Tendency of the change of the concentration in reservoir beds】
- Will radioactive contamination continue to accumulate in reservoirs due to the inflow of radioactive cesium from rivers? 【Effect of the upstream dam on 137Cs accumulation in the riservoir beds】
- How much does additional internal dose exposure would be if we assume highest concentration during the fire event was exposed? How harmful for the health?
