Radioactivity and Air Dose Rate
(2019)
QI heard lichens tend to capture cesium. What is the mechanism like?(1)
AThinking that some metabolites produced by lichens are involved in the retention of radioactive cesium, JAEA and the National Museum of Nature and Science successfully determined the formation energy of complexes*1 formed by cesium and five metabolites produced by lichens such as Parmotrema tinctorum and Flavoparmelia caperata found in Fukushima Prefecture by utilizing quantum chemical calculations*2, for the first time ever.
As a result, it was found that cesium complexation energy varied depending on the metabolite and the pH environment.
This result suggests that lichens secrete metabolites with different properties to send “the right man for the job” for improved cesium retention energy.
*1 Complex: a collective term for molecules created by bonding of an atom and a molecule or of different molecules.
*2 Quantum chemical calculation: calculation of electron orbits of atoms and molecules based on quantum physics.
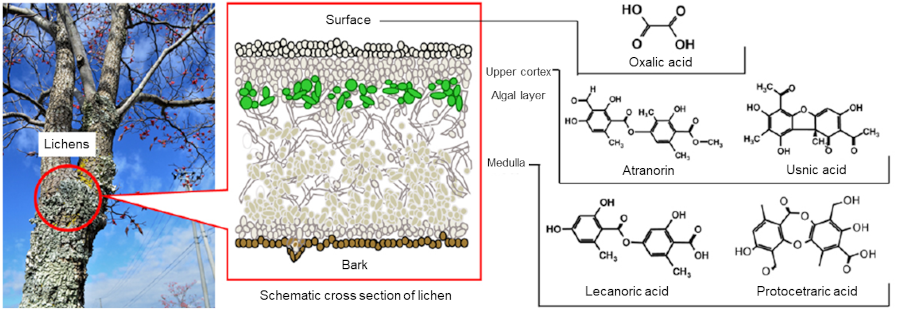
Fig. 1 Lichens growing on a tree (Flavoparmelia caperata, seen as green epiphyte on the surface of the trunk in the photograph), and schematic illustration of the internal structure of a lichen
Lichens such as Parmotrema tinctorum and Flavoparmelia caperata secrete different metabolites from different layers of their bodies. According to the calculation, metabolites in the upper cortex*3 and in the medulla*4 form complexes strongly bound with alkali metal elements*5 including cesium when the environment is alkaline and neutral, respectively.
*3 Upper cortex: the surface of lichen’s body (thallus). A protective layer consisting of condensed algal cells, present at the top of the algal layer (Fig. 1).
*4 Medulla: the internal part of lichen’s body. A layer consisting of mycelia loosely intertwined (Fig. 1).
*5 Alkali metal elements: elements in the Group 1A of the periodic table (excluding hydrogen), namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
For the purpose of elucidating the mechanism of long-term retention of radioactive cesium by lichens, JAEA and the National Museum of Nature and Science conducted a study for determining complexation energy between lichen metabolites and cesium using a quantum chemical calculation technique.
Lichen is a collective term for symbiosis between a fungus and an alga. Lichens are unique organisms that slowly grow on the surface of relatively stable matter, such as rocks, trunks (Fig. 1), house walls, and concrete. Lichens have been known to exhibit a long-term radioactive cesium retention property, and biologists and atmospheric chemists have been trying to unravel the retention mechanism. In this study, we thought that some metabolites generated by algae inside a lichen strongly adsorb radioactive cesium and form complexes, enabling long-term retention of radioactive cesium.
To support this hypothesis, for the first time ever, we conducted a study for determining complexation energy between alkali metal elements including cesium and metabolites generated by Parmotrema tinctorum and Flavoparmelia caperata, which were found to be retaining radioactive cesium in Fukushima, by applying a quantum chemical calculation technique. During the study, we found that complexes were unstable and changed their structures at room temperature and in water, and therefore we had to determine the structures of multiple complexes efficiently and accurately as they emerged. To address this issue, we adopted a technique to divide calculations into two stages (1st stage: low-accuracy high-speed calculation to obtain multiple structures a complex can take; 2nd stage: high-accuracy parallel calculations using a super computer for the multiple structures obtained in the 1st stage calculation) and successfully improved the calculation speed significantly. As a result, the calculations completed in only several days, which would take a much greater amount of time otherwise. This newly developed technique has a wide range of applications, such as monitoring of in-vivo dynamics of drugs (organic molecules), not limited to lichen metabolites.
It was found from the calculation results that atranorin and usnic acid generated in the upper cortex (Fig. 1, right) formed complexes strongly bound with alkali metal elements in alkaline conditions, because the upper cortex is closer to the outer world and tends to become alkaline for relatively high concentrations of alkali metal elements. Meanwhile, lecanoric acid and protocetraric acid, metabolites generated in the medulla (Fig. 1, right), formed strongly bound complexes in neutral conditions with less alkali metal element concentrations.
These results indicate that lichens are able to retain alkali metal elements including cesium from the upper cortex to medulla by generating various metabolites in the layers of their bodies according to the environment the layers are in. Until today, it has been difficult to experimentally observe where radioactive cesium is retained inside the body of lichens and which metabolites are involved in the retention. We believe this study clarified for the first time ever that the mechanism of radioactive cesium retention by lichens, in which multiple metabolites are involved in formation of cesium complexes depending on the environment given.
