Information on treated water and decommissioning
(2018)
QWhat is a cesium ball?
AParticles that are glassy with a size of about 1 μm to 1 mm and contain a relatively large amount of cesium, which are difficult to dissolve in water, are called insoluble cesium particles. The cesium ball is a common name for the smaller and spherical ones. In the accident at TEPCO's Fukushima Daiichi Nuclear Power Station, it was confirmed that this cesium ball was released during a limited period in the early stages of the accident.。
Emission of insoluble cesium (Cs) particles was detected in environmental samples collected during the early stage of the TEPCO’s Fukushima Daiichi NPS (1F) accident. Particles with diameters of 1 µm to 1 mm were particular ejecta of the accident.
There were at least two types of particles of which the main element is silicate (Fig.1)
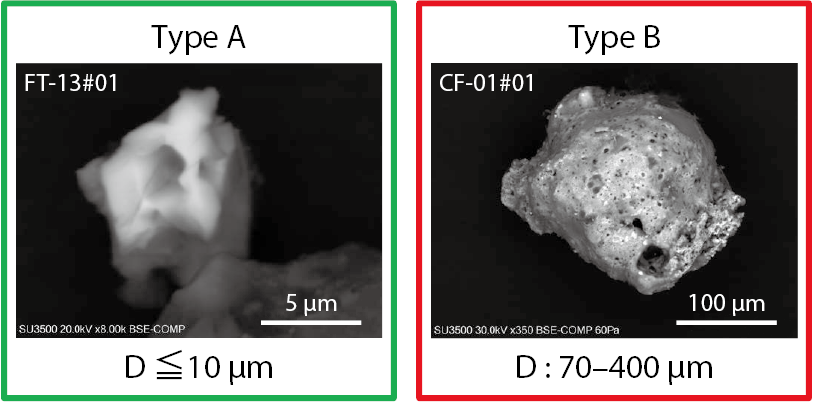
Fig.1 Two types of radioactive insoluble cesium particles
Type A is a few µm in diameter and has been observed extensively in eastern Japan, including the Tokyo Metropolitan Area.
Type B has a larger mean diameter and is found mainly in the vicinity of the 1F.
This silicate makes the particles insoluble in water. Type A is a few µm in diameter and contains a small amount of radioactive Cs within it. However, the concentration of Cs is so large that characteristic X-rays can be detected using energy-dispersive X-ray spectrometry (EDS), which has a much higher detection limit than other methods. By contrast, Type B particles are hundreds of microns in size and can be see with the naked eye, but the concentration of Cs is lower than in Type A. The radioactivity concentration per unit volume is called the specific radioactivity, but in order to clarify the above relationship, when two kinds of particles are illustrated by the particle volume and the Cs concentration contained, the inclination of Type B is smaller than that of Type A. The results of previous studies (● and ● in Fig.2) of particles considered to have been released at the same time (midnight on March 14) as Type A also agreed with specific radioactivity relationships.
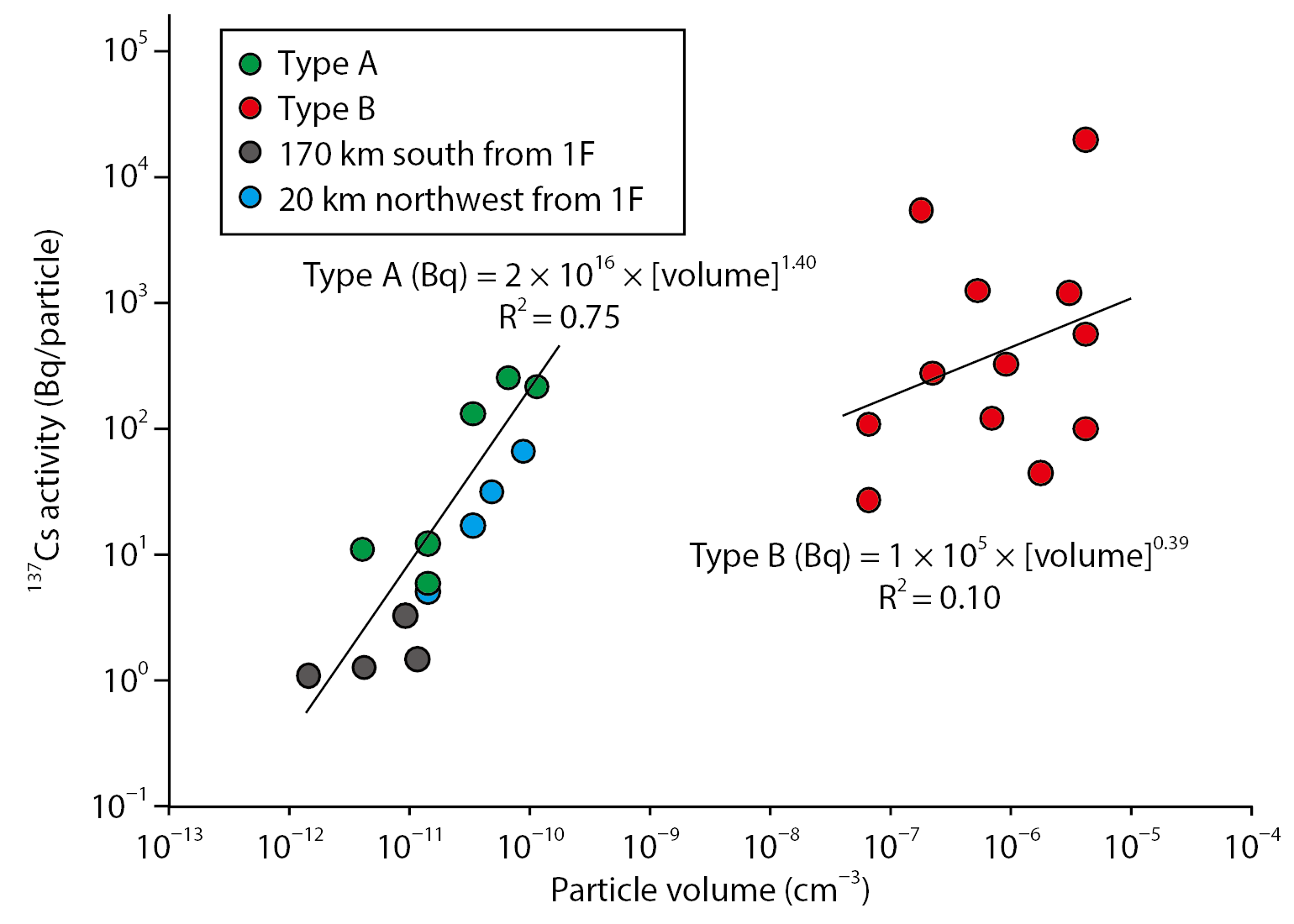
Fig.2 Particle volume vs. cesium concentration
The specific radioactivity of Type A is larger than that of Type B.
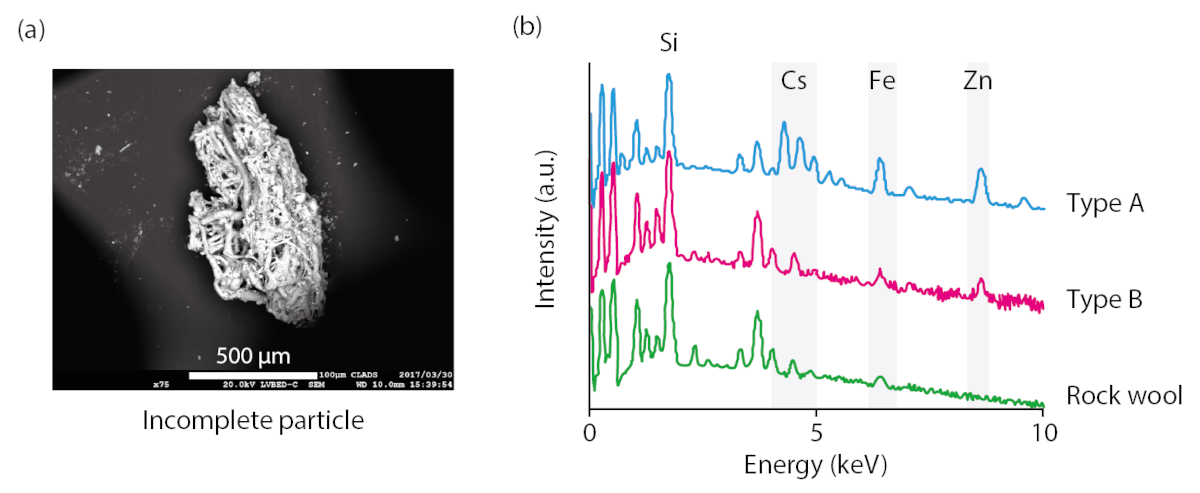
Fig.3 Intermediate type-B particles and the results of energy-dispersive X-ray spectrometry (EDS)
(a) Attached fiber-silicate materials and melted fibers were observed. (b) EDS spectra of the standard type of heat insulator and the Type B particle were consistent.
Type-B particles with low specific radioactivity were confirmed to have been released by the 1F Unit 1 reactor hydrogen explosion on March 12. Moreover, Type-B particles were deposited over a limited region north of the polluted nuclear plant immediately after the explosion took place. Observing Type B, fibrous silicate compounds were confirmed to adhere surface (Fig.3(a)). Based on this discovery, we investigated the silicate compounds used around the reactor building. The elemental composition of the heat-insulating material used inside the building and the constituent elements of Type B nearly agreed (Fig.3(b)). This result shows that cesium filled in the reactor building, adsorbed onto fibrous insulation made of silicate compounds, shrunk, spread due to the hydrogen-explosion heat and the blast, and scattered as Type-B particles into the environment. On the other hand, Type-A particles, whose generation process has many unknown features due to their high specific activity, are a subject for further study.
Related articles
- Where can I find information on decommissioning?
- Is it possible to visualize radiations? Also, is there a way to understand the situation of contamination inside the Fukushima Daiichi NPS buildings?
- What happened to the inside of the reactor pressure vessel and storage vessel after the accident?
- What information is needed to safely retrieve fuel debris?
