Radioactivity and Air Dose Rate
(2021)
QDoes cesium stick to other than clay minerals?
AFocusing on the large grain size fraction containing a large amount of Cs, it was found that not only mica minerals, which have been suggested to be strongly adsorbed in past studies, but also apparent colored minerals contain the same amount of radioactive Cs. I did. It was also found that radioactive Cs is also present in apparent colorless minerals that are generally considered to be difficult to adsorb. This is because the surface of colored minerals and colorless minerals is transformed into clay minerals by weathering, and it is possible that these are involved in the adsorption of Cs.
Most of the radiocesium (137Cs) emitted from the TEPCO’s Fukushima Daiichi NPS accident was deposited into the river system, where it was transported by soil particles and redistributed in the downstream area. Identifying the dominant mineral species that have adsorbed 137Cs is necessary to predict the elution from a mineral to river water and sedimentation behavior of 137Cs. In this study, the relationship between the dominant mineral species that adsorb 137Cs and the behavior of 137Cs moving downstream from the upstream reservoir within the Tomioka river basin is clarified.
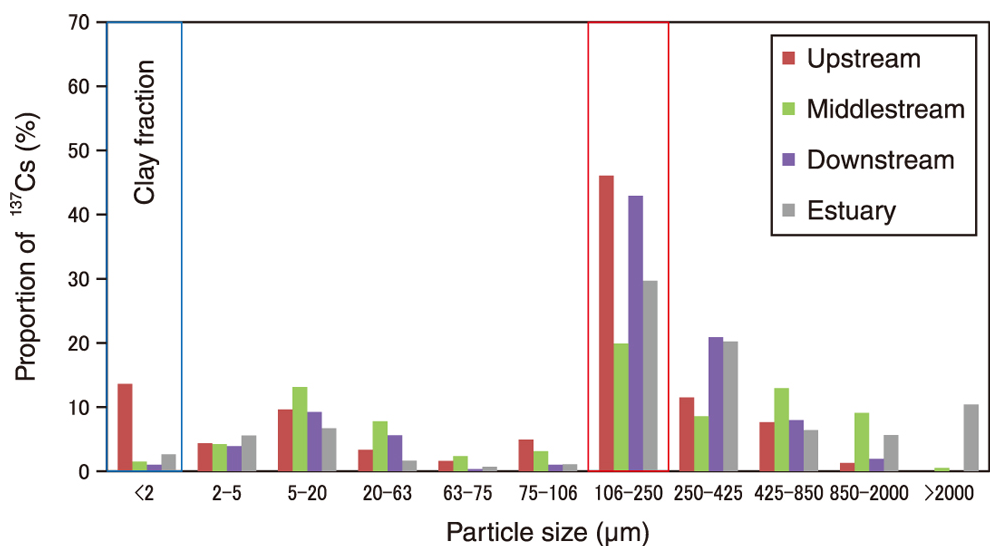
Fig.1 Ratio of 137Cs in each size fraction to the total 137Cs in the bulk sediment sampled from the upstream reservoir to the estuary
Fine sand (106–250 μm: red box in figure) contained the highest 137Cs quantities. Unlike in previous studies, coarse fractions contained high levels of 137Cs.
Some sediment samples were collected in the riverbed from the upstream to the downstream. The collected sediments were divided into 11 fractions by their size (i.e., according to their Wentworth grain size) via sedimentation and centrifugation. The radioactivity of 137Cs in each fraction was determined using a germanium semiconductor detector. The contribution of 137Cs in each size fraction to the total 137Cs concentration in the bulk sediment was then calculated using the weight frequency of each fraction. The results indicate that the fine sand fraction (106–250 μm) in the riverbed sediments contained the largest 137Cs quantities (Fig.1) due to the higher weight proportion of fine sand to other fractions. Thus, mineral species in the coarse-grain fraction except clay minerals strongly adsorbed 137Cs.
The mineral species were then separated using morphological observations from the size-fractioned sediments to identify which mineral species strongly adsorb 137Cs. Using a microscope and X-ray diffractometer, the mineral species were separated and identified as mafic minerals (hornblende, augite, and magnetite), as mica (vermiculite), as felsic minerals (quartz and feldspars) (Figs.2(a)–(c)). Micas, which have been reported to have adsorbed 137Cs, and mafic minerals were demonstrated to have an equivalent ability to adsorb 137Cs.
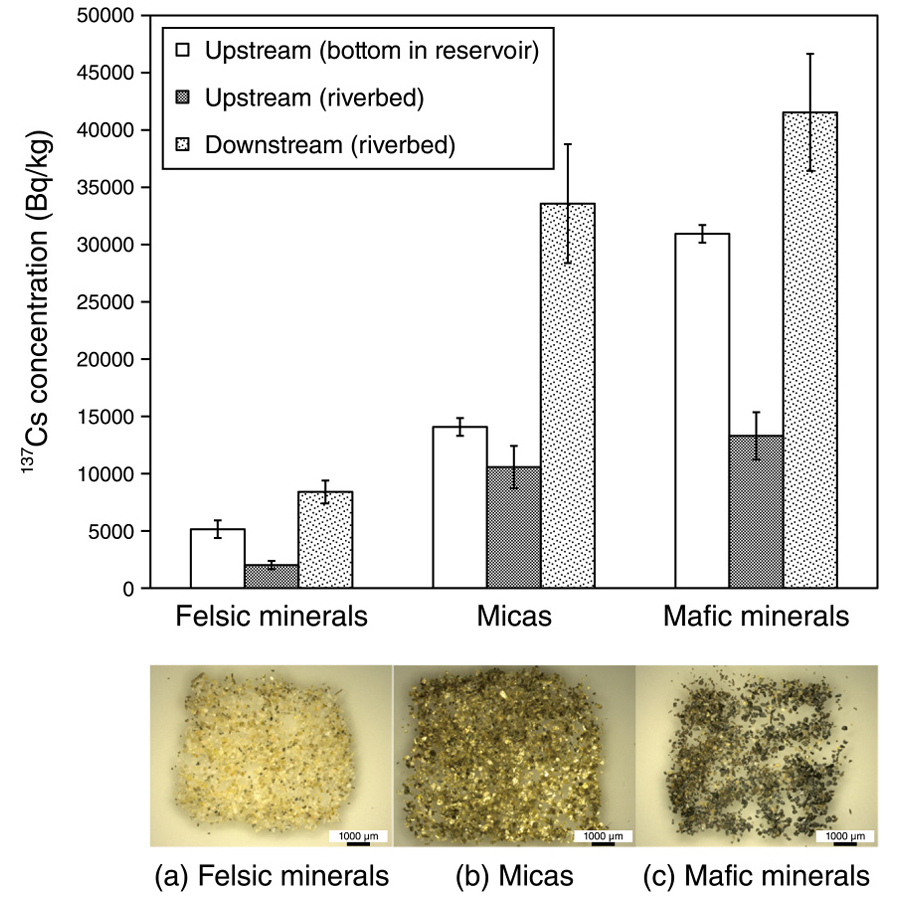
Fig.2 137Cs concentrations in each mineral and separated mineral obtained from the fine sand fraction at the riverbed and reservoir
The fine sand was separated into (a) felsic minerals, (b) micas, and (c) mafic minerals. Although previous studies have indicated that 137Cs is poorly sorbed onto mafic and felsic minerals, 137Cs was sorbed these minerals.
The felsic minerals, which have been reported as poor adsorbers of 137Cs, also contained 137Cs. Scanning electron microscopy (SEM) demonstrated that the surface of the feldspars and hornblende has many fine particles and a flaky structure due to weathering (Fig.3), which may promote the sorption of 137Cs. This study may contribute to clarifying the sorption and desorption mechanisms of 137Cs on minerals for evaluating the transport of 137Cs.
This work was performed in collaboration with Niigata University, entitled “Mineralogical study on behavior of radiocesium in environment”.
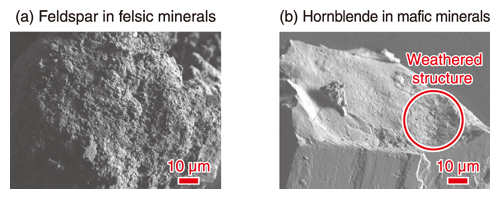
Fig.3 Morphological characteristic of the representative minerals hand-picked from the fine sand fraction using SEM
The surface of (a) feldspar in felsic minerals and (b) hornblende in mafic minerals were likely to be weathered.
